Oxidative phosphorylation is a process involving a flow of electrons through the electron transport chain, a series of proteins and electron carriers within the mitochondrial membrane. This flow of electrons allows the electron transport chain to pump protons to one side of the mitochondrial membrane. As the protons build up, they create a proton-motive force, a type of electrochemical pressure. This pressure is relived through specialized protein complexes, which capture the energy of the protons as they flow to the other side of the membrane. The energy is then used to bond a phosphate group to the molecule adenosine diphosphate (ADP), creating adenosine triphosphate (ATP). This completes the process of oxidative phosphorylation.
Steps of Oxidative Phosphorylation
Before the Electron Transport Chain
For the electron transport chain to be able to pump protons to one side of the mitochondrial inner membrane, it must first have a source of those electrons and protons. There are several cellular processes which lead to the oxidation (“burning”) of various cellular food sources. These processes include glycolysis, the citric acid cycle, the fatty acid beta-oxidation metabolism, and the oxidation of amino acids.
All of these processes involve the transfer of electrons and protons to coenzymes. The most common coenzymes are nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD). NAD can be reduced with electrons and a proton to become NADH, while FAD can take on two protons and four electrons to become FADH2. These coenzymes can bind to the proteins of the electron transport chain, and transfer their electrons and protons. This becomes the first stage in the electron transport chain.
Within the Electron Transport Chain
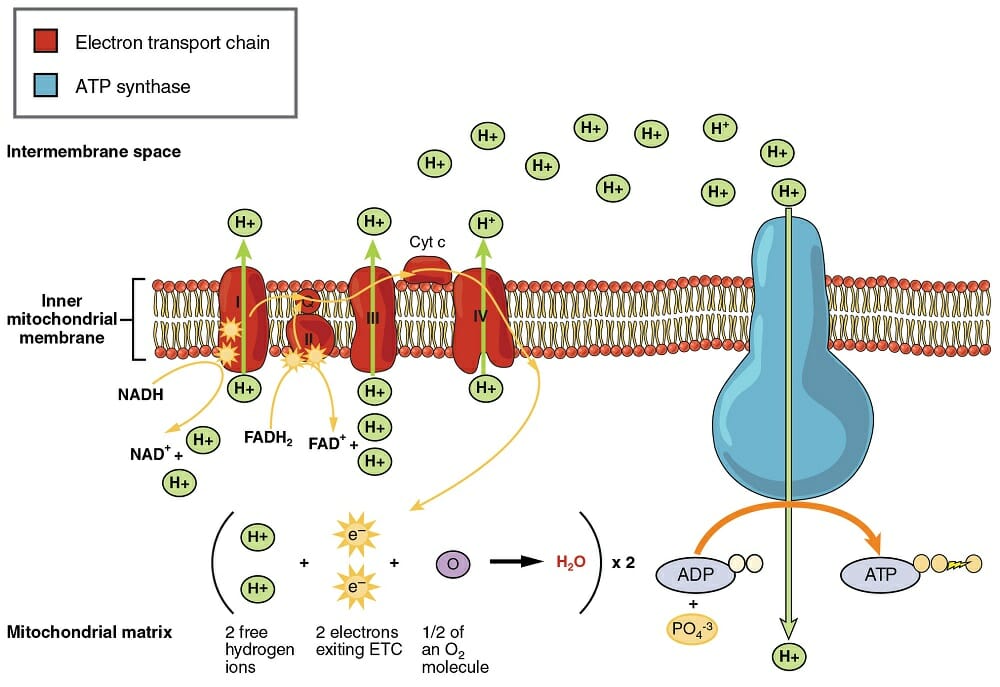
The electron transport chain consists of four protein complexes, simply named complex I, complex II, complex III, and complex IV. Each complex is designed to receive electrons from a coenzyme or one of the other complexes in the chain. The actions each complex takes can be seen in the image below.
Complex I is responsible for relieving NADH of its hydrogen and electrons. The energy received by taking the electrons allows complex I to pump the hydrogen atom through the inner mitochondrial membrane, which concentrates hydrogens in the intermembrane space. The electrons are then passed to coenzyme Q (CoQ). CoQ can take on hydrogens and electrons, and can be reduced to CoQH2. The coenzyme transfers the electrons to complex III.
Meanwhile, complex II is also receiving electrons and protons. These come from FADH2, from the citric acid cycle. Complex II relieves FADH2 of its electrons, and passes them to CoQ. The coenzyme passes them to complex III, which now receives electrons and their energy from two sources. This allows complex III to pump large amounts of hydrogen across the membrane. Cytochrome c (Cyt c) allows the electrons to be passed to complex IV, the final complex in the electron transport chain. This complex passes the electrons to oxygen molecules, where they bind with hydrogens to produce water. With the final bit of energy, another proton is passed through the membrane.
ATP Synthesis
At this point, the electron transport chain has built up a large number of hydrogen ions in the intermembrane space. It did this with the energy it received through passing electrons through a series of energy releasing reactions. The final step of oxidative phosphorylation is the production of ATP, or the process of phosphorylation.
This process takes place in a complex called ATP synthase. This large complex uses the proton-motive force to attach phosphate groups to ADP molecules. Because there are so many protons built up in the intermembrane space, they want to push their way to the other side. ATP synthase uses this energy to undergo a conformational change. In doing so, it forces the ATD and phosphate group together, and reduces the energy they need to bond. ATP can then go on to fuel reactions all over the cell, when it is exported from the mitochondria.
The Electron Transport Chain Within Oxidative Phosphorylation
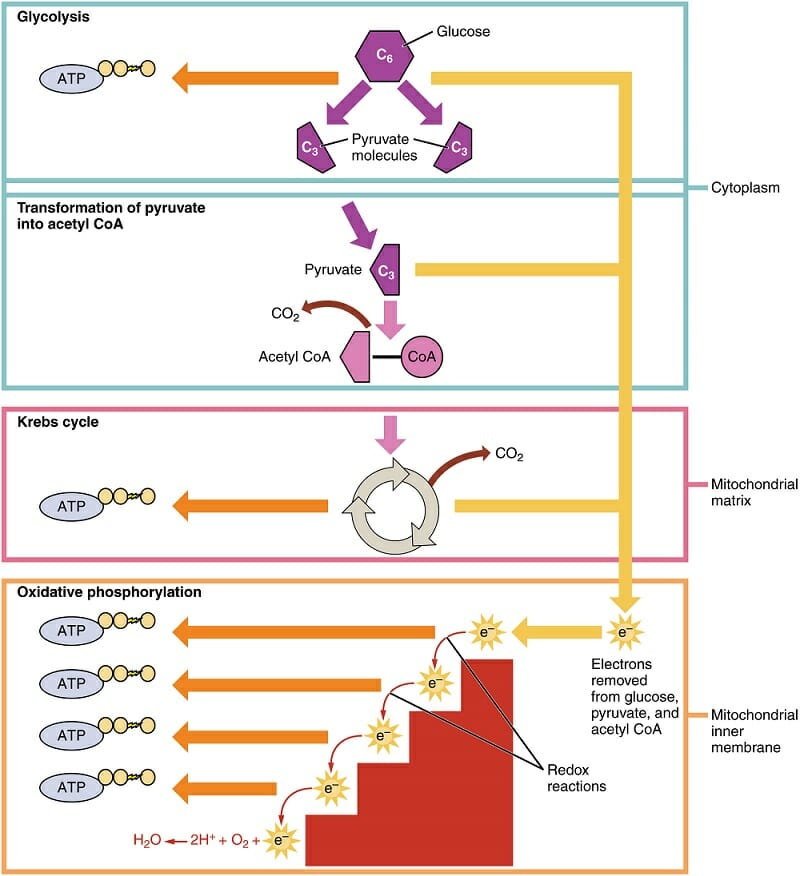
Oxidative phosphorylation is part of a larger system, cellular respiration. The 4 steps of cellular respiration can be seen in the image below. The first step occurs outside of the mitochondria. This involves the breakdown of glucose, lipids, or amino acids. This step is symbolized here with “Glycolysis” only. Remember that there are other ways to generate pyruvate and intermediates the Krebs cycle (citric acid cycle).
The remaining steps take place within the mitochondria. The yellow lines in the image represent the generation of reduced coenzymes, or molecules which are carrying electrons. While some ATP is generated during glycolysis and the citric acid cycle, the majority is generated through oxidative phosphorylation. The electron transport chain is symbolized by the red staircase, representing the successive release of energy from the electrons. The orange arrows represent ATP synthase, which creates ATP through the proton-motive force.
Oxidative Phosphorylation within Cellular Respiration
Therefore, the electron transport chain is a part of oxidative phosphorylation, which itself is the last stage of cellular respiration. The truly interesting thing about these processes is that they are conserved across evolution. The electron transport chain can be observed in the most basic of organisms. Any eukaryote (cell with organelles), has mitochondria and therefore uses this exact same method to produce ATP. Even plants, which are often considered so different than animals, rely on the same process of oxidative phosphorylation.
Interestingly, the process of photophosphorylation is very similar to oxidative phosphorylation. This process is used in photosynthesis. However, instead of using oxygen to create water, it uses water to create oxygen. Basically the opposite of oxidative phosphorylation, photosynthesis uses an electron transport chain of its own to carry energy from sunlight into the bonds of sugar molecules. The plant can then use these molecules to feed other cells within its body. Just as an animal would, it breaks the glucose into pyruvate, and the pyruvate enters the mitochondria and eventually undergoes oxidative phosphorylation powered by the electron transport chain.
Quiz
1. Which of the following is a true statement?
A. Oxidative phosphorylation and the electron transport chain are unrelated
B. Oxidative phosphorylation drives the electron transport chain
C. Oxidative phosphorylation relies on the electron transport chain
2. What would happen to a cell if there was no electron transport chain?
A. The cell would have no energy
B. The cell would fall apart
C. The cell would have less energy
3. As a scientist in your laboratory, you extract the mitochondria from your own cell, and from the cells of your favorite house plant. You put each mitochondria in a small dish, surrounded with pyruvate. You measure how much ATP each mitochondria makes. What do you expect?
A. The animal mitochondria will make more ATP
B. The plant mitochondria will make more ATP
C. They will produce roughly the same amount of ATP
References
- Lodish, H., Berk, A., Kaiser, C. A., Krieger, M., Scott, M. P., Bretscher, A., . . . Matsudaira, P. (2008). Molecular Cell Biology (6th ed.). New York: W.H. Freeman and Company.
- Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company.
- Widmaier, E. P., Raff, H., & Strang, K. T. (2008). Vander’s Human Physiology: The Mechanisms of Body Function (11th ed.). Boston: McGraw-Hill Higher Education.
Electron Transport Chain and Oxidative Phosphorylation
No comments:
Post a Comment