Integral Protein Definition
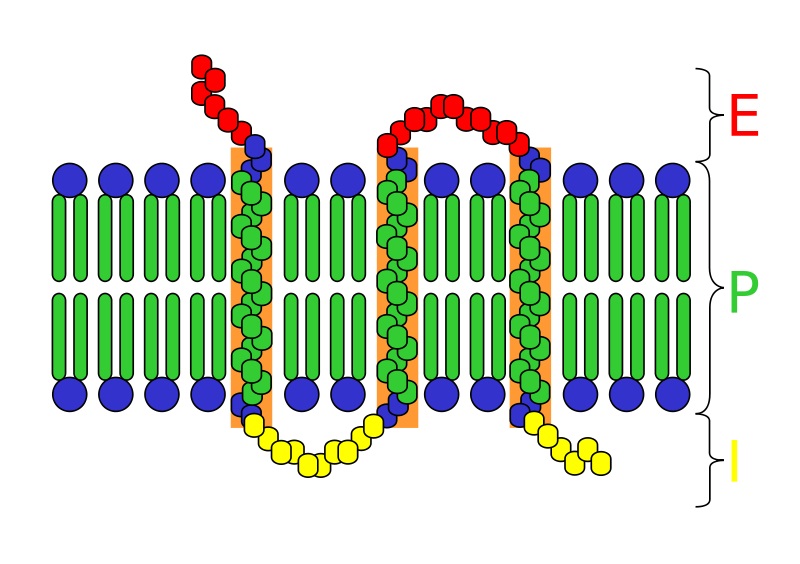
An integral protein, sometimes referred to as an integral membrane protein, is any protein which has a special functional region for the purpose of securing its position within the cellular membrane. In other words, an integral protein locks itself into the cellular membrane. It does so with regions of specific amino acids which are attracted to the middle of the plasma membrane. A typical integral protein can be seen in the image below.
The integral protein seen here crosses the plasma membrane (P) several times. This is not always the case, some integral proteins have only a single region which extends into the hydrophobic internal layer of the plasma membrane. The region of the protein seen in green is also hydrophobic. The positive influence of these non-polar interactions and the negative force of trying to push into a region filled with water keep integral proteins in place. Besides this basic function caused by the similar structure of all integral proteins, a single integral protein can take part in many different reactions.
An integral protein can be compared to a peripheral protein. A peripheral protein is often attached to the plasma membrane, but only to the heads of the phospholipid molecules. Most can detach easily, and are not really bound within the membrane. An integral protein, because of the chemistry of the environment around it, can never leave the plasma membrane. Sometimes a peripheral protein and integral protein will work in conjunction to complete a task.
Integral Protein Function
The basic function of at least one part of every integral protein is to attach the protein to a plasma membrane. This membrane may be the plasma membrane surrounding the mitochondria, or the inner membrane of the mitochondria. They are present on the outermost cell wall, as well as the nuclear envelope, which surround the nucleus and binds the DNA. There is an integral protein associated with every living plasma membrane, and most cells include hundreds, if not thousands of them.
The ultimate function of each integral protein varies by organism, organelle, and even by location along a microscopic piece of plasma membrane. One integral protein may function as messenger, transferring a signal between the extracellular space and the cytosol. Many integral proteins like this are used in the reception of hormones, and the transfer of their messages.
Some integral membrane proteins are part of large complexes of proteins, responsible for a number of reactions which take place across a membrane. ATP synthase, for example, is the multi-protein complex which produces ATP in living organisms from plants to humans. It resides on the inner mitochondrial membrane. Here, the electron transport chain has amassed ions on one side of the membrane, creating a gradient. ATP synthase uses the pressure of this gradient like a hydro-electric dam, and uses the energy provided to produce ATP.
A different integral protein may not extend all the way through the plasma membrane. Instead, these integral proteins may need to be bound to a membrane so that their product is easy to expel. Some of the proteins responsible for producing neurotransmitters operate in this way. This allows the product to be amassed where it is needed most, at the very tips of the neurons where the signal can be released.
Integral Protein Structure
While the structure of an integral protein outside of the plasma membrane binding region can vary widely based on function, there are only three common themes of binding to the plasma membrane within living cells that we currently know of. The first two involve the sequence of amino acids which makes up the protein, and the third involves a modification to the protein after it is created which gives it a lipid-based anchor within the plasma membrane.
The Alpha Helix
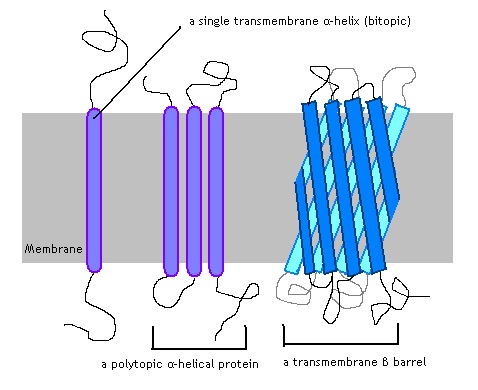
The alpha-helix is a shape produced by a certain chain of amino acids which looks exactly as its name implies. The interactions between the amino acids next to each other make a downward and inward bend, creating a structure similar to a spiral staircase. Alpha helices tend to be non-polar, giving them a distinct advantage to staying bound within the hydrophobic tail-region of the membrane. A transmembrane alpha helix spans all the way through the membrane. An integral protein may only have one region of alpha helix, as shown in the far left of the image below.
Many other proteins employ several alpha helices, which span the membrane. This allows for the creation of a protein channel, or a hole in the plasma membrane which allows various substances to pass. Common among bacteria is the third image, the beta barrel.
The Beta Barrel
A beta sheet is a complexly folded chain of amino acids which forms a flattened, rigid sheet. Like the alpha helix, it is one of the principle shapes a chain of amino acids can take on. When many beta sheets extend through the membrane, creating a pore, the structure is called a beta barrel. The outsides of the beta sheets have hydrophobic residues, and the integral protein can be locked into the plasma membrane. Like the transmembrane alpha helix, the beta barrel requires the correct sequence of amino acids for the integral protein to maintain contact with the membrane.
The Lipid Anchor
A lipid anchor is a non-polar, hydrophobic attachment to some proteins which allows it to be embedded within the plasma membrane. Instead of being coded into the genetic code of the protein, the protein itself is modified through a different process. Through a biochemical reaction, a fatty acid or other lipid is covalently bonded to the protein itself, usually at one end. The lipid is then used in the constitution of the plasma membrane, where it becomes trapped by its nature with the other lipids of the tail regions of the phospholipids. An integral protein with a lipid anchor is not picture in the above image.
Quiz
1. Which of the following is the defining feature of an integral protein?
A. Portion which binds to the hydrophobic region of the plasma membrane
B. Attaching to the plasma membrane in any way
C. Conducting enzyme reactions near the membrane
2. A scientist in the laboratory has learned how to separate integral proteins from the plasma membrane. He simple puts cells in a solution containing detergent, like dish soap, and the proteins are extracted from the membrane. What must detergent be doing to the proteins to extract them whole?
A. Destroying the bonds of their amino acids
B. Replacing the bonds of the plasma membranes with those of the detergent molecules
C. Physically cutting the integral protein from the membrane
3. By only looking at the genetic code, what is one way to distinguish an integral protein from a protein which does not bind to the membrane?
A. There is no way to tell simply by looking at the genetics
B. Look at how many A’s vs T’s there are in the code
C. Look for signs of alpha helices and beta barrels
References
- Bruice, P. Y. (2011). Organic Chemistry (6th ed.). Boston: Prentice Hall.
- Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company.
- Widmaier, E. P., Raff, H., & Strang, K. T. (2008). Vander’s Human Physiology: The Mechanisms of Body Function (11th ed.). Boston: McGraw-Hill Higher Education.
Integral Protein
No comments:
Post a Comment