Cholinergic Definition
Cholinergic is a term used to refer to the molecule acetylcholine. It is usually employed to define neurons, receptors or synapses that use acetylcholine. For instance, a cholinergic neuron is a neuron that releases acetylcholine, and a cholinergic receptor is a receptor to which acetylcholine binds. Acetylcholine is a signal molecule in the nervous system that is used by nerve cells to transfer information. It is widely present in the peripheral nervous system, which is involved in contracting skeletal and smooth muscle and in dilating blood vessels, among other functions. Acetylcholine plays a major role at the neuromuscular junction, i.e. at the joint between nerve cells and muscle. Additionally, acetylcholine is also present in the central nervous system, where it plays a role in cognitive processes such as memory, learning and arousal.
Unsurprisingly, its role in numerous processes of the peripheral and the central nervous systems have made the cholinergic system a target in the treatment of multiple. In turn, several cholinergic drugs have been developed for clinical as well as for cosmetic purposes. For example, some cholinergic drugs are used to treat severe muscle spasms, others to slow down the progression of Alzheimer’s disease and others to reduce wrinkles. However, in addition to the therapeutic and cosmetic effects, cholinergic drugs can also induce a series of side effects, including paralysis of the autonomic nervous system.
Acetylcholine Function
Acetylcholine is present in the peripheral and in the central nervous systems. In the peripheral nervous system, acetylcholine is largely implicated in muscle movement and in other functions such as blood vessel dilation. In the central nervous system, it is involved in cognitive functions.
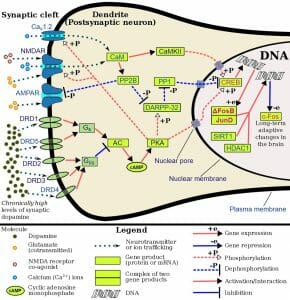
Acetylcholine acts by binding to cholinergic receptors, the two main types of which are muscarinic and nicotinic. Muscarinic acetycholine receptors (mAChR) are G protein-coupled receptors (GPCR) that modulate the activity of the cell by activating cellular mechanisms involving second messengers. There are five identified types known as M1 to M5. M1, M3 and M5 muscarinic receptors are usually excitatory and are of the Gq type; thus, they exert their function by activating phospholipase C (PLC), which in turn activates the IP3 signal transduction cascade, allowing extracellular calcium to enter the cell. M2 and M4 receptors are usually inhibitory and are of the Gi or Go type, i.e. they act by reducing cyclic AMP (cAMP) in the cell. Nicotinic acetylcholine receptors (nAChR), the other main type, are ligand-gated ion channels that, when activated by acetylcholine, directly allow ions to enter (e.g. sodium) or exit the cell (e.g. potassium).
Acetylcholine in the Peripheral Nervous System
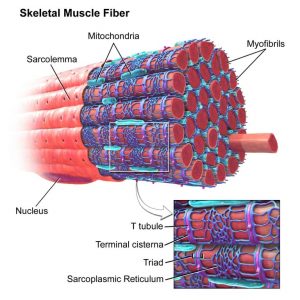
Acetylcholine is a major player at the neuromuscular junction. The neuromuscular junction is the site where a nerve cell (a neuron) and skeletal muscle (the kind of muscle that is voluntarily contracted) are connected. For a muscle to contract, the brain sends electrochemical signals from one neuron to another, until an action potential (electrical signal) is generated at the motor neuron, which is the neuron that contacts the muscle fiber. At the neuromuscular junction, acetylcholine is released by the motor neuron into the synaptic cleft, which then binds to nicotinic acetylcholine receptors present on the muscle fiber cell. Nicotinic acetylcholine receptors allow sodium to enter the muscle cell, after which a series of intracellular signals lead to the contraction of the muscle. Anomalies in peripheral cholinergic transmission have been linked to motor disorders such as myasthenia gravis, a disorder characterized by fatigue and muscle weakness.
Acetylcholine is also a widely used neurotransmitter in the autonomic nervous system—a part of the peripheral nervous system involved in the control of unconscious and involuntary bodily functions. Specifically, acetylcholine is released by neurons from central nervous system that project to neurons of the autonomic nervous system, the latter of which detect acetylcholine through nicotinic acetylcholine receptors. These neurons in turn project to body parts that do not belong to the nervous system, such as the gastrointestinal tract. In some cases, acetylcholine is also released at this junction between the peripheral nervous system and other body parts.
Acetylcholine in the Central Nervous System
In the central nervous system, cholinergic activity is related to arousal, awareness, learning, memory, attention and reward, among others. Not surprisingly, abnormal cholinergic transmission has been associated with Alzheimer’s disease, a neurodegenerative condition characterized by memory loss.
Cholinergic Drugs
The involvement of acetylcholine in diseases of the nervous system has naturally made the cholinergic system a target for therapeutic purposes. Drugs that activate (agonists) or inactivate (antagonists) acetylcholine receptors, as well as drugs that modulate cholinergic activity by facilitating or preventing the production, release or degradation of acetylcholine, have been developed with the aim to treat several neuropsychiatric conditions.
Agonists
Acetylcholine has a very short life: it does not last long in the bloodstream because it is degraded very fast. Therefore, acetylcholine itself is not used as a drug, but instead similar compounds that activate acetylcholine receptors are employed to activate them. These similar compounds that bind to and activate acetylcholine receptors are known as acetylcholine agonists.
An example of an agonist is pilocarpine, which activates muscarinic receptors and is usually applied in the pupil of the eye to treat a neurodegenerative disease that causes blindness called glaucoma. Another example of an agonist is nicotine, found in tobacco.
Antagonists
Many cholinergic drugs are acetylcholine receptor antagonists, which block acetylcholine receptors. Some antagonists are atropine, scopolamine, hexamethonium and trimethaphan. Atropine and scopolamine inactivate muscarinic receptors and are used to suppress bodily secretions (e.g. tears or mucus) and to relax smooth muscle (e.g. muscles in the gastrointestinal tract) during anesthesia, and to treat motion sickness. Hexamethonium and trimethaphan block nicotinic receptors and are used to reduce high blood pressure. Other agents that block nicotinic receptors are used because of their effects at the neuromuscular junction; these agents prevent skeletal muscles from contracting and are often employed during surgery to keep patients from making involuntary movements.
Other Drugs
In addition to cholinergic agonists and antagonists, other drugs can modulate acetylcholine activity by increasing or decreasing its production, release or degradation. For instance, inactivating acetylcholine transferase, which is an enzyme that breaks down acetylcholine, is employed to increase the levels of acetylcholine and to treat myasthenia gravis, a neuromuscular disorder. Similar drugs such as neostigmine and pyridostigmine do not cross the blood-brain barrier and are consequently employed to exert their effect at the neuromuscular junction and contract skeletal muscle.
Nonetheless, anticholinergics—drugs that reduce or block the effects of acetylcholine—are more widely used to treat numerous conditions. Some of these are involuntary movements, gastrointestinal disorders, incontinence and Parkinson’s disease. Another compound that blocks the release of acetylcholine is botulinum toxin—an agent produced by a type of bacterium—which paralyzes the skeletal muscle so that the organism is no longer able to move and which can even cause death. When applied locally, botulinum toxin relaxes muscles and is consequently utilized to treat severe muscle spasms. The same compound is used to reduce wrinkles by relaxing the muscles and skin; we know this under the trade name Botox.
Cholinergic Effects
The effects of activating cholinergic receptors include muscle contraction, heart rate deceleration, constriction of the iris (miosis) and of the lens, mucus secretion and broncho-constriction. Conversely, the effects of inactivating cholinergic receptors include muscle relaxation, heart rate acceleration, pupil dilation (mydriasis) and lens flattening (cyclopegia), dryness of the upper airway (of the respiratory system), inhibition of tear production, urine retention, mouth dryness, slowing down of mucociliary activity in the respiratory tract, constipation and muscle relaxation (skeletal muscle and smooth muscle).
Cholinergic Side Effects
Cholinergic drugs can help treat some disorders and ameliorate symptoms but they also have negative side effects. Most cholinergic drugs are anticholinergics, i.e. they reduce or block the effects of acetylcholine. For instance, the acetylcholine antagonists hexamethonium and trimethaphan, used to treat high blood pressure, can produce paralysis of the autonomic nervous system, producing effects such as blurred vision and inability to urinate. Anticholinergics in general can cause a raise in body temperature because they reduce the amount of sweating; they can also induce drowsiness, hallucinations, confusion, dry mouth, constipation, difficulty urinating and memory deficits. In older people, they can cause confusion, memory loss and cognitive decay. Mixing anticholinergics with alcohol have similar side effects as overdosing with anticholinergics, which include dizziness, fever, confusion, accelerated heart rate, trouble breathing, hallucinations, unconsciousness and even death. Caution is therefore warranted when taking cholinergic drugs.
Quiz
1. Where does acetylcholine play a major role?
A. At the neuromuscular junction.
B. At the corticospinal tract.
C. In all the peripheral nervous system.
D. In all the central nervous system.
E. All of the above.
Answer to Question #1
A is correct. Acetylcholine is involved in some but not in all processes in the peripheral and central nervous systems. It does however play a major role at the neuromuscular junction, where motor neurons meet muscle fibers.
2. What are some effects of inactivating cholinergic receptors?
A. Heart rate deceleration and muscle relaxation.
B. Heart rate acceleration and muscle spasms.
C. Pupil dilation and muscle spasms.
D. Urine retention, dryness of the upper respiratory tract and muscle relaxation.
Answer to Question #2
D is correct. Blocking acetylcholine receptors results in multiple effects two of which are urine retention, dryness of the upper respiratory tract and muscle relaxation.
3. Which disorders are treated with acetylcholine antagonists or anticholinergic drugs?
A. Gastrointestinal disorders, bipolar disorder and motion sickness.
B. Gastrointestinal disorders, Parkinson’s disease and high blood pressure.
C. Glaucoma, Alzheimer’s disease, Parkinson’s disease and low blood pressure.
D. Glaucoma, Alzheimer’s disease and high blood pressure.
Answer to Question #3
B is correct. Due to its effects on the gastrointestinal tract, on muscle movements and on blood vessel dilation, blocking the effects of acetylcholine with antagonists or anticholinergic drugs is used to treat multiple clinical conditions, some of which are gastrointestinal disorders, Parkinson’s disease and high blood pressure.
References
- Anderson, K.-E. (2011). Muscarinic Acetylcholine Receptors in the Urinary Tract. In: Urinary Tract. Berlin-Heidelberg: Springer.
- Changeux, J.-P., Edelstein, S.J. (2005). Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition. New York: Odile Jacob.
- Encyclopedia Britannica (2014, December 18). Cholinergic drug. Retrieved from: https://www.britannica.com/science/cholinergic-drug
Cholinergic