Deuterostome Definition
The Deuterostomes are a clade of animals that undergo deuterostomy during their embryonic development. They are a sister-clade of the Protostomes, and the two together with the Xenacoelomorpha form the major group of animals called the Bilateria—a major group animals which display bilateral symmetry and are mostly triploblastic.
Deuterostomy
During embryonic development, the fused gametes from the male and female—the sperm and the egg—form the zygote.
In order to develop, the zygote undergoes a process called cleavage. Cleavage involves splitting into multiple cells called blastomeres, and results in a dense ball of these cells called a morula. In deuterostomy, radial cleavage occurs, whereby the blastomeres are arranged along a central axis and is characterized by several tiers of cells stacked on top of each other. Radial cleavage is one of the defining features of the deuterostome development, contrasting the spiral cleavage that is typical of the protostomes. Additionally, most of the deuterostomes display indeterminate cleavage, in which the developmental fate of each cell is not predetermined in the embryo and therefore each cell has the ability to develop into a complete embryo if isolated.
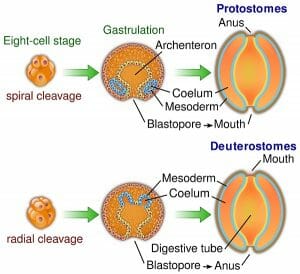
The image shows the defining differences between deuterostome and protostome embryonic development.
The blastula is the resulting structure, consisting of at least 128 cells surrounding a cavity of mainly empty space, called the blastocoel. Through a process called gastrulation, the cells of the blastula are reorganized to form the three primary germ layers of the gastrula that are present in all triploblastic organisms. Gastrulation begins with a small indentation in the blastula called the blastopore, the cells of which migrate to the opposite end of the embryonic structure, establishing the endoderm layer; the endoderm eventually gives rise to the digestive system.
In deuterostomes, the first cavity formed by the blastopore ends up as the organism’s anus, while the mouth is formed secondarily on the opposite side. This is the next major distinction between deuterostomes and protostomes; the protostomes form the mouth from the primary cavity and the anus second.
It is useful to note that the two names are derived from the Greek proto- “first” and deutero- “second”, and stoma meaning “mouth”. The deuterostomes develop a “second-mouth”.
In many egg-laying deuterostomes the peripheral layer of cells in the gastrula forms the ectoderm, which ultimately gives rise to the epidermis (the skin and hair) and the nervous system. In between the endoderm and the ectoderm is the mesoderm, which ends up as connective tissues, skeletal system, blood, the heart and kidneys and muscle.
In mammal development the outer layer of the blastula equivalent—the blastocyst—becomes the placenta and the inner cells give rise to the three primary germ layers.
Types of Deuterostome
The Deuterostomes can be taxonomically grouped into three clades.
Echinodermata
The echinoderms are a group of marine animals, which although are radially symmetrical in adult life, display bilateral symmetry in their larval stage and are thus classed within the Bilateria.
The echinoderms have anendoskeleton just below the skin made from calcium carbonate which provides rigidity and protection. Additionally, they have a hydrostatic skeleton—a fluid filled cavity present in many developed animals called the coelom, supported by hydrostatic pressure to allow movement.
Many echinoderms have structures called ‘tube feet’, which they use to grasp substrate in order to move, as well as for feeding and respiration. Predatory species use the tube feet to pry open bivalves and then feed by extruding the stomach out of the mouth to digest the prey. Non-predatory species use the tube feet for suspension feeding, whereby they flick food to their cilia, which then pass the food into the mouth.
The Echinoderms are separated into six taxonomic classes.
- Crinoidea—Feather stars and sea lilies
- Asteroidea—Sea stars
- Ophiuroidea—Brittle stars
- Echinoidea—Sea urchins and sand dollars
- Holothuroidea & Concentricycloidea—Sea cucumbers & Sea Daisies

The image above shows an illustrative example of echinoderms from each taxonomic class.
Chordates
The chordates are a phylum of animals within the deuterostomes, which have the following common similarities:
- A notochord. A flexible, supportive rod, made from material similar to cartilage. In the vertebrates this is replaced by the vertebral column during development.
- A hollow dorsal nerve chord—This is formed from the ectoderm and runs the length of the body. In vertebrates, this makes up the central nervous system.
- A post-anal tail. A tail that extends beyond the anus in at least some point of their development.
- Pharyngeal gill slits in at least some point of their development. These are openings within the throat that allow the animal to breathe underwater. In marine organisms these become functioning gills, and in terrestrial animals they are modified for alternative functions.
Note that: All vertebrates are chordates—not all chordates are vertebrates.
The chordates can be separated into 3 subphyla:
The Cephalochordata
These cephalochordates are small invertebrate marine animals known as lancelets. They are simple fish-like organisms, which live with their tails buried in the sand and employ a filter feeding system. They are most likely the closest link between the chordates and other simple organisms.
The Urochordata
The urochordata includes the tunicates—also known as ‘sea squirts’. These invertebrate organisms are usually sessile and possess a U-shapes gut and two siphons, which allows them to take in food. The gills slits are modified in the adult form to allow filter feeding.

The image shows sea squirts, members of the urochordata which intake food and through their siphons (the visible holes).
The Vertebrata
The vertebrata is the largest subphylum within the chordates and the most morphologically complex. In addition to the typical characteristics of chordates, the vertebrates all posses a skull or cranium, which encases the brain and a backbone or vertebral column, which protects the dorsal nerve chord and internal organs as well as providing support.
They are also notable for the following evolutionary developments:
- A hinged jaw, which allows the animal to capture food in a highly effective way.
- The amniotic egg, containing a protective inner membrane through which gases and nutrients can be transferred to the embryo during development.
- Limbs, either as fins or evolved into legs for improved movement ability.
- The evolution of the pharyngeal gills into lungs.
The vertebrates are separated into seven extant taxonomic classes:
- Agnatha—The hagfish and lampreys. Although they have a skull and basic vertebrae, these lack a jaw and vertebral column.
- Condrichthyes—The sharks, skates, rays and sawfish. These have gills and an endoskeleton made from cartilage.
- Osteichthyes—The bony fish. These have gills, an endoskeleton made from bone, and a ‘swim bladder’, which helps with depth control.
The ‘tetrapods’ are four-limbed vertebrates within the chordates:
- Amphibia—Frogs, toads and salamanders. These are both marine and terrestrial organisms. Although in the adult form most of them have lungs, they can also breathe through their skin.
- Reptilia—Turtles, snakes, crocodiles, lizards. These are ectothermic animals with scales and lungs.
- Aves—Birds. These are endothermic animals with feathers and beaks.
- Mammalia—These are endothermic amniotes with the defining characteristics of: 1)hair, which aids in insulation 2)mammary glands for producing milk 3)a neocortex, which allows complex brain function 4)three middle ear bones for enhanced hearing sensitivity 5)internal fertilization
Within the mammalia are the Eutherians or ‘placental mammals’; a group which includes the primates, such as monkeys and humans, cetaceans (whales and dolphins), rodents, cats, dogs and most other animals that are familiar to us.
The class Mammalia also includes the marsupials such as kangaroos, wombats and opossums, in which the offspring are born under-developed and complete development within a maternal pouch on the mother’s stomach.
Further included are the Monotremes, of which only the duck billed platypus and echidna species are extant. The monotremes are mammals that lay hard shelled eggs, additionally they lack nipples, so secrete milk through specialized hair follicles.
Hemichordata
The hemichordates are marine deuterostomes, which are characterized by a body that is comprised of three distinct sections: The anterior (front) prosome, the middle mesosome and the posterior (back end) metasome.
These are generally worm-like filter feeders, deposit feeders and detritivores and are considered to be the closest existing relatives to the vertebrates. Like other chordate deuterostomes, the hemichordates have pharyngeal gill slits and most have a dorsal nerve chord, although they lack the notochord.
They are divided into two classes:
- Enteropneusta — The acorn worms. These can reproduce both through sexual and a-sexual reproduction.
- Pterobranchia
Related Biology Terms
- Protostomes – A clade of animals in which spiral cleavage occurs during embryonic development and the blastopore develops into the mouth.
- Coelom – The fluid filled cavity present in most animals, which surrounds the digestive tracts and other organs.
- Phylogenetic Tree – A diagram representing the evolutionary relationships between living organisms.
- Bilateral Symmetry – A characteristic of the Bilateria Clade, in which the two sides of the body are mirror images of each other.
Quiz
1. Which form of cleavage is characteristic of all deuterostomes?
A. Radial cleavage
B. Spiral cleavage
C. Indeterminate cleavage
D. Rotational cleavage
Answer to Question #1
A is correct. All deuterostomes undergo radial cleavage in embryonic development. However, only some deuterostomes undergo indeterminate cleavage and only mammals undergo rotational cleavage. Spiral cleavage is characteristic of the protostomes.
2. Which of the following is a feature not associated with the chordates?
A. Dorsal nerve chord
B. Hydrostatic skeleton
C. Pharyngeal gill slits
D. Amniotic egg
Answer to Question #2
B is correct. The hydrostatic skeleton is the endoskeleton present within the echinoderms, as well as some other soft-bodied invertebrates.
3. In the deuterostomes, what is the fate of the blastopore?
A. The mesoderm
B. The mouth
C. The anus
D. The placenta
Answer to Question #3
C is correct. In the deuterostomes development, the blastopore eventually gives rise to the anus, while the mouth develops from the second cavity. ‘Deuterostome’ literally means ‘second-mouth’.