Sucrose Definition
Sucrose, commonly known as “table sugar” or “cane sugar”, is a carbohydrate formed from the combination of glucose and fructose. Glucose is the simple carbohydrate formed as a result of photosynthesis. Fructose is nearly identical, except for the location of a double-bonded oxygen. They are both six-carbon molecules, but fructose has a slightly different configuration. When the two combine, they become sucrose.
Plants use sucrose as a storage molecule. For quick energy, cells may store the sugar for later use. If far too much is accumulated, plants may begin to combine the complex sugars like sucrose into even large and denser molecules, like starches. These molecules, and oily lipids, are the main storage chemicals used by plants. In turn, animals eat these sugars and starches, break them back down into glucose, and use the energy within the bonds of glucose to power our cells.
Sucrose has been an important sugar for humans because it is easy extracted from plants such as sugar cane and sugar beets. These plants tend to store an excess of sugar, and from this we produce the majority of the sugar that we use. Even most “natural” sweeteners, which claim to be healthier than sucrose, are simply a different version of glucose combined in a different manner by plants.
Sucrose Structure
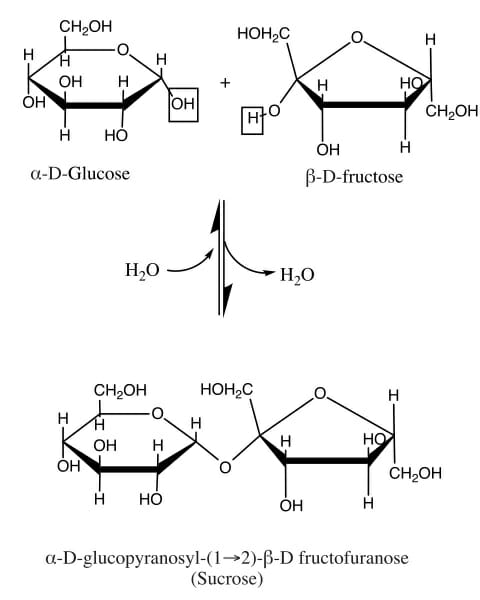
As mentioned above, sucrose is disaccharide, or a molecule made of two monosaccharides. Glucose and fructose are both monosaccharides, but together they make the disaccharide sucrose. This is an important process for the storage and compression of energy. Plants do this to make it easier to transport large amounts of energy, via sucrose. This process can be seen in the following image.
Glucose is seen on the left. Glucose is known as an aldose, meaning the carbonyl group (carbon double bonded to an oxygen) is found at the end of the chain of carbons. When the molecule creates a ring back on itself, it forms a 6-sided ring. Fructose, on the other hand, is a ketose. This means that the carbonyl group is found in the middle of the middle of the molecule. In this case, it forces fructose into a five-sided ring.
In a plant creating sucrose, an enzyme comes along to smash these two rings together, and extract a molecule of water. This process is called a condensation reaction, and forms a glycosidic bond between the two molecules. As you can see in the image, the reaction can also go the other way. To dissolve sucrose into fructose and glucose, a molecule of water can be added back in. This is what happens to sucrose as you digest it.
Sucrose Uses
Sucrose is the most common form of carbohydrate used to transport carbon within a plant. Sucrose is able to be dissolved into water, while maintaining a stable structure. Sucrose can then be exported by plant cells into the phloem, the special vascular tissue designed to transport sugars. From the cells in which it was produces, the sucrose travels through the intercellular spaces within the leaf. It arrives at the vascular bundle, where specialized cells pump it into the phloem. The xylem, or vascular tube which carries water, adds small amounts of water to the phloem to keep the sugar mixture from solidifying. The sucrose mixture then makes its way down the phloem, arriving at cells in the stem and roots which have no chloroplasts and rely on the leaves for energy.
The sucrose is absorbed into these cells, and enzymes begin breaking the sucrose back into its constituent parts. The six-carbon glucose and fructose can be broken down into 3-carbon molecules, which are imported into the mitochondria, where they go through the citric acid cycle (AKA the Krebs Cycle). This process reduces coenzymes, which are then used in oxidative phosphorylation to create ATP. The energy within the bonds of ATP can power many of the reactions these cells need to complete in order to maintain the stem and roots.
Likewise, all other life on Earth is dependent upon sucrose and other carbs produced by plants. Sucrose was one of the first substances to be extracted from plants on a mass-scale, creating the white table sugar we know today. These sugars are extracted and purified from large crops, including sugar cane and sugar beets. To extract the sugar, the plants are usually boiled or heated, releasing the sugar. “Sugar in the Raw” is sugar which has not been treated further, while white table sugar undergoes more purification.
Quiz
1. Which of the following terms describes sucrose?
A. Disaccharide
B. Monosaccharide
C. Ketose
2. Which of the following is NOT a use of sucrose?
A. Sweetener
B. Information storage
C. Carbon-transport
3. The items listed below all store energy within their bonds. Which of the following molecules stores the MOST energy?
A. Glucose
B. Sucrose
C. Starch
References
- Jones Jr., J. B. (2005). Hydroponics: A Practical Guide for the Soilless Grower (2nd ed.). Boca Raton: CRC Press.
- McMahon, M. J., Kofranek, A. M., & Rubatzky, V. E. (2011). Plant Science: Growth, Development, and Utilization of Cultivated Plants (5th ed.). Boston: Prentince Hall.
- Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company.
Sucrose
No comments:
Post a Comment