Definition of Endergonic Reaction
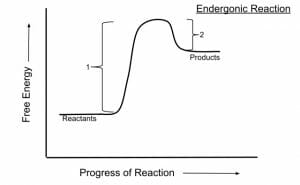
An endergonic reaction is a reaction in which energy is absorbed. In chemistry terms, this means that the net change in free energy is positive – there is more energy in the system at the end of the reaction than at the beginning of it.
Because endergonic reactions involve a gain in energy, that energy has to be supplied from an outside source in order for the reaction to occur.
In biology, organisms use endergonic reactions to store energy from outside sources. Photosynthesis, which uses the energy of sunlight to create sugars, is an endergonic reaction. So is fatty acid anabolism, in which the energy from food is stored in fat molecules.
In general, reactions that involve creating new chemical bonds are endergonic. The chemical bonds “store” the reaction energy until they are broken, at which point some of the energy that was put into the initial reaction is released.
This is the principle on which the metabolism of glucose, fatty acids, and other biological fuels is based. Energy from sunlight or another source that was used to create the chemical bonds in sugars, proteins, or fats is liberated when those bonds are broken through processes like glycolysis and cellular respiration.
In general, metabolic reactions that involve creating chemical bonds are called “anabolic” reactions. Metabolic reactions that involve breaking bonds to release energy are called “catabolic.”
It is this movement of energy through chemical bonds which allows life to exist. The endergonic reactions of photosynthesis and chemosynthesis allow creatures at the bottom of the energy pyramid to survive – and to feed organisms like ourselves, who get their energy by breaking down sugars and fats to liberate that stored energy.
Function of Endergonic Reactions
Endergonic reactions have two important purposes in biology. One is to release energy stored in food molecules, allowing organisms to survive without harvesting all their energy directly from sunlight.
The other purpose is to create the building blocks of life: DNA, RNA, proteins, and all the other building blocks of cells must be created through reactions that form new bonds between chemical building blocks. These bond-building reactions are generally endergonic.
Organisms need energy to grow because it actually takes energy to produce new materials. For plants, this may mean the sugars, lipids, and nucleic acids that their leaves are made of; for humans, it means the lipids of our cell walls, the protein in our muscles, and of course the DNA in our cells.
In most cases, the energy required to build new cells comes from ATP. ATP is a storage molecule for the energy of glucose; which ultimately comes, of course, from the sun through photosynthesizing plants.
Examples of Endergonic Reactions
DNA/RNA Synthesis
DNA and RNA synthesis are fascinating because they do not use ATP the same way more endergonic reactions do. You may recall that DNA has four bases – A, T, C, and G. Well, the “A” base pair stands for adenosine – the same as the “A” in “ATP!”
Rather than being expended and then regenerated during DNA synthesis, ATP is one of the building materials. The process starts out with trisophosphates of each of the base pairs: ATP, TTP, CTP, and GTP.
When DNA polymerase moves one of these nucleotide triphosphates into position to attach to the growing DNA strand, one of the nucleotide’s phosphate groups breaks off – and is replaced by the formation of a new bond between the nucleotide and the DNA strand!
Somewhere down the line, this process does require energy and the use of ATP – all of the nucleotides have to have phosphate groups attached to them, so that these phosphate groups can store the energy needed to create a bond between the nucleotide and the DNA strand.
But unlike many catabolic reactions, this one does not simply turn ATP into ADP and send it back to get a new phosphate group. In this one, the ATP, TTP, GTP, and CTP stay as part of the DNA strand forever, until the strand is broken down!
Protein Synthesis
Protein synthesis is a more typical example of how living things move energy, and add it to reactions to allow new chemical bonds to form.
During protein synthesis, a variety of enzymes and ribozymes work together to complete the steps necessary to add an amino acid to a growing protein. In all, about five ATP must be consumed to add a single amino acid to a growing protein. That means that for every glucose molecule that is metabolized, about six amino acids could be added to a protein!
This process is immensely costly for bacteria; for E. coli cells, about 95% of all the ATP they make is used for protein synthesis.
This investment pays off handsomely in the long run, since proteins such as enzymes can drastically lower the activation energy required for thousands of subsequent chemical reactions. But for organisms that cannot perform cellular respiration, the energy budget is tight!
The proteins that are made with the energy from ATP allow our metabolisms, muscles, and even our brains and sensory organs to function. And it’s important to remember that this energy is supplied to us in the food we eat – which ultimately, at the bottom of the energy pyramid, comes from photosynthesis!
Fatty Acid Synthesis
Fatty acid synthesis uses both ATP and another energy-carrying molecule – NADPH – to supply energy to create fatty acids.
Making a fatty acid takes a great deal of energy; it can take 7 ATPs and 14 NADPH to add two carbon molecules to a fatty acid chain, and some fatty acids can have up to 26 carbons!
But fatty acids, just like proteins, are necessary for an organism to function and grow; they make up most of the cell and intracellular membranes, as well as serving other purposes.
If the fatty acid is being created for the purpose of energy storage, most of that energy will be stored and can be accessed by the organism later, if its reserves of ATP and sugar run low!
Quiz
1. Which of the following is LEAST likely to be an endergonic reaction?
A. The synthesis of a starch from many molecules of sugar.
B. The synthesis of a protein from many amino acids.
C. The catabolism of a fat into its single-carbon components.
D. None of the above.
2. Why doesn’t DNA polymerase use ATP?
A. Because DNA synthesis is an exergonic reaction.
B. It uses NADPH as an energy source instead.
C. It does use ATP – and other nucleotide triphosphates, which supply their own energy to the synthesis reaction.
D. None of the above.
3. Which of the following is NOT true of protein synthesis?
A. Bacteria must metabolize more sugar in order to pay the energy “cost” of protein synthesis, because they can’t perform cellular respiration.
B. Protein synthesis is essential to the creation of enzymes, which are proteins.
C. It releases more energy than it expends.
D. None of the above.
References
- MacNaught, A. D., & Wilkinson, A. (1997). Compendium of chemical terminology: IUPAC Recommendations. Oxford: Blackwell Science.
- (n.d.). Retrieved April 29, 2017, from http://webprojects.oit.ncsu.edu/project/bio183de/Black/cellcycle/cellcycle_scripts.htm
- Piques, M., Schulze, W. X., Höhne, M., Usadel, B., Gibon, Y., Rohwer, J., & Stitt, M. (2009). Ribosome and transcript copy numbers, polysome occupancy and enzyme dynamics in Arabidopsis. Molecular Systems Biology, 5. doi:10.1038/msb.2009.68
- Deis, F. (n.d.). Does protein synthesis take any energy (ATP)? Retrieved April 29, 2017, from https://www.quora.com/Does-protein-synthesis-take-any-energy-ATP
Endergonic Reaction
No comments:
Post a Comment