Lactate Dehydrogenase Function
Lactate dehydrogenase (LDH) is an enzyme found in most living organisms. It is the enzyme responsible for the conversion of pyruvate, the end product of glycolysis, into lactic acid. With this conversion, the molecule also uses a unit of the energy transferring molecule NADH, releasing the hydrogen to produce NAD+. This conversion is necessary when a cell has little to no oxygen.
While the process of oxidative phosphorylation in the mitochondria produces the most energy, some energy is produced by the breakdown of glucose into pyruvate. This process, glycolysis, requires NAD+, but produces ATP. The cell can use this small amount of ATP to keep the cell operating until oxygen returns. Instead of using the pyruvate in the Krebs cycle, the pyruvate is converted to lactic acid. When oxygen returns, lactate dehydrogenase can reverse its enzymatic function. This direction creates pyruvate, which can be broken down with oxygen in the mitochondria to produce and abundance of ATP.
Lactate dehydrogenase is present within all the cells of your body, and works to maintain homeostasis in the absence of oxygen. While this may seem like a rare event, even simple exercise can lead to oxygen deprivation in certain tissues. Lactate dehydrogenase allows these tissues to continue to produce energy, without oxygen. This causes lactic acid buildup in your muscles and tissues, and is partially responsible for the “burn” felt while you exercise strenuously. When oxygen returns to the muscles, the lactic acid will be converted back into pyruvate and the sensation will cease.
Lactate dehydrogenase is also an important commercial enzyme. It is the culprit behind sour milk, and as such has been used to create many dairy dishes, including cottage cheese, kefir, and yogurt. Some brewers and wineries even use lactate dehydrogenase to create a distinct sour flavor in their beer or wine. The enzyme works the same in or out of a cell, and converts pyruvate into lactic acid. Lactic acid has a distinctly sour taste.
Structure of Lactate Dehydrogenase
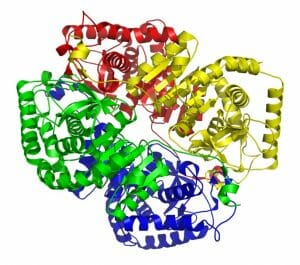
Lactate dehydrogenase consists of 4 different subunits, which work as a cohesive unit. These 4 subunits can come in different forms, and are coded by different genes. In the human body, there are 5 different isoforms, or versions, of lactate dehydrogenase. These different versions are found in different body tissues, which can help doctors identify where the lactate dehydrogenase come from.
For instance, LDH-1 (lactate dehydrogenase-1) is found in the heart, blood cells and brain. LDH-3 is only found in the lungs. Doctors can recognize the different versions of lactate dehydrogenase by the different subunits they consist of. LDH-5 can be seen in the image below. The different colors represent the individual protein units which made up the enzyme. Remember that these proteins function as a single unit in the enzyme.
Lactate Dehydrogenase Test
The lactate dehydrogenase test can be used to detect tissue damage. Further, because of the differentiation of different types of lactate dehydrogenase, doctors can use a lactate dehydrogenase test to determine where and how much damage is taking place in the body.
For instance, someone who recently had a heart attack will have elevated levels of lactate dehydrogenase in their blood. The enzyme gets released from the tissues which were damaged. In this case, the damaged heart tissue would have released the lactate dehydrogenase. Doctors can test the enzyme, and determine that it is indeed LDH-1, the form of lactate dehydrogenase found in the heart tissue. Depending on the levels found in the blood or spinal fluid, doctors can estimate how much heart tissue was damaged and how long ago the incident occurred.
The lactate dehydrogenase test can be used to search for a number of other ailments. Typically it is used to monitor or diagnose internal tissue damage, monitor a condition causing damage, or evaluate the treatment of certain cancers. While the test is often used in conjunction with many other indicators, high levels of lactate dehydrogenase in the blood or spinal fluid often indicate tissue damage. However, even vigorous exercise can elevate the levels of the enzyme. Often more important than the level of the enzyme are the symptoms and type of lactate dehydrogenase found in the blood.
Typically, newborns have the highest levels of the enzyme in their blood, with up to 450 units per liter (U/L). Infants have slightly less, maxing out at somewhere around 250 U/L. Children, who are actively growing, typically have the least, with a maximum of 170 U/L. Adults will tend to have close to 200 U/L. When levels go above this, it is an indication of tissue damage. In the spinal fluid, the typical level is much lower, between 40 and 70 U/L. Elevated levels in the spinal fluid can indicate bacterial infections of the spinal cavity and brain. The treatment of certain cancers is measured in part by the amount and type of lactate dehydrogenase in the system. This can indicate if the chemotherapy and radiation are targeting the right tissue types.
Besides cancer and heart failure, this test can be used to identify hypothyroidism, anemia, pre-eclampsia, meningitis, encephalitis, HIV, and liver or lung disease. The many isoforms of lactate dehydrogenase make it an excellent tool for identifying which tissues are being damaged by a particular disease. This can lead to a more confident diagnosis.
Quiz
1. You receive a blood test. This test indicated that your levels of LDH-3 are significantly higher than they should be. What does this indicate?
A. You might have lung damage
B. You had a heart attack
C. Your pancreas is failing
2. The production of lactic acid by lactate dehydrogenase is a form of fermentation. The production of ethanol, the alcohol in beer and wine and liquor, is also a fermentation product. Where do these processes differ?
A. Ethanol production does not use pyruvate
B. They use different enzymes
C. Ethanol production happens only with oxygen
3. HIV is a virus, which causes the body to lose immune defenses, a condition known as AIDS. In this state, the body is at increased risk for infection. HIV itself does not raise lactate dehydrogenase levels. Why do doctors of HIV patients monitor their lactate dehydrogenase levels?
A. It is an unnecessary test
B. They are watching for tissue decay
C. It is an early sign of infection in someone without an immune system
References
- Bruice, P. Y. (2011). Organic Chemistry (6th ed.). Boston: Prentice Hall.
- Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company.
Lactate Dehydrogenase
No comments:
Post a Comment