Heme Definition
A heme is an organic, ring-shaped molecule. Due to its special structure, a heme is capable of holding, or “hosting” an iron molecule. A heme is made from 4 pyrroles, which are small pentagon-shaped molecules made from 4 carbons and 1 nitrogen. Four pyrroles together form a tetrapyrrole. If the tetrapyrrole has substitutions on the side chains which allow it to hold a metal ion, it is called a porphyrin. Thus, a heme is an iron-holding porphyrin.
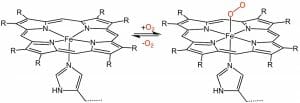
The iron molecule in a heme is held in place by the balanced attractive forces of the four nitrogen molecules. The nitrogen molecules all point toward the inside of the larger ring they create. The double and single bonds which connect the pyrroles are arranged evenly, so that the electrons stay balanced and the entire molecule remains stable. This makes it an aromatic molecule. A heme molecule can be seen below.
Hemes are used for two known reasons: to carry oxygen and to transport or store electrons. In the above image, you can see how gaseous oxygen can reversibly bind to the heme complex. Organisms use the heme molecule, in complex with specially-shaped proteins, to transport oxygen and move electrons. These special proteins, like hemoglobin and myoglobin, are made to help the heme complex hold or release oxygen at the appropriate times.
Heme is named for the Greek derivative for “blood”, which is where it was first identified. The red color of blood is produced by the heme and iron ion interacting to absorb other colors and only reflect red. A somewhat different effect is seen in chlorophyll. Chlorophyll is a porphyrin complex used in photosynthesis. Instead of iron, chlorophyll houses a magnesium ion, and chlorophyll has different side chains than a heme group. This produces the green color of plants, rather than the reds and purples of blood.
Heme Structure
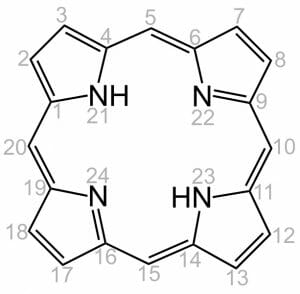
Like all porphyrins, heme has a base structure of a large ring of four pyrroles. This base molecule, only seen rarely as an intermediate in nature, is called porphin. There are many different forms of heme, which correspond to the many functions it must serve in an organism. Specific proteins use the varying side chains to attach to, and they change the properties of the heme. However, the base structure is always the same. It is the tetrapyrrole shown below.
The numbers on the molecules indicate points in which the molecule may receive substitutions and be modified for a specific use. Differences in the side-chains attached to carbons 3, 8, and 18 constitute the difference between some of the most common heme groups. For instance, hemoglobin and myoglobin both carry Heme B. Heme B carries oxygen, and the proteins it is attached to help it release the oxygen at the appropriate time. Heme A, on the other hand, works in the electron transport chain as part of cytochrome c. This means that it is involved in transporting proteins and catalyzing reactions. The only difference between the two molecules are the side-chains attached at carbons 3 and 18. The side chain on carbon 8 remains the same.
Heme Function
Heme has two understood functions. It can bind gases, such as oxygen, and transport them throughout an organism. Special proteins then force the heme to release its oxygen at the appropriate time. A good example of a protein of this type is hemoglobin. Hemoglobin is found in all blood cells, attached to the cell membrane, exposing the heme group to the blood plasma. Thus, when the blood cells pass through the lungs, they bind up as much oxygen as the iron in the heme can handle.
The blood cells then travel to various parts of the body, such as the muscles. These cells are actively using up oxygen and releasing carbon dioxide as a byproduct. Carbon dioxide forms an acid in the blood plasma, lowering the pH of the blood. Like all proteins, hemoglobin reacts to changes in pH by changing shape. This change in shape forces the oxygen off of the heme complex, releasing the oxygen into the blood plasma. The oxygen diffuses into the muscle cells, where it is bound by myoglobin and transported to the mitochondria to be used. Myoglobin also has a heme group, but it operates in a different way so that oxygen remains bound until reaching the mitochondria.
The second function of hemes, holding electrons and facilitating reactions in the electron transport chain, occurs in all organisms. During oxidative phosphorylation in the mitochondrial membrane, electrons must be passed down a series of reactions, which slowly extract their energy before depositing them in water and carbon dioxide. The energy gained is stored in the bonds of the molecule ATP, which most organisms use as a primary source of energy. The heme groups in these cytochromes are different than those in hemoglobin, for they have different functions and bind to different proteins.
Quiz
1. As mentioned in the article, heme is found in virtually all living organisms. What does this suggest about life on Earth?
A. All life developed heme in a common ancestor
B. Heme is an important primary food source
C. Life on Earth is simple
2. What would happen if you replaced all of the myoglobin in an organism with hemoglobin?
A. Nothing, the organism would still function properly
B. The organism would not receive enough oxygen in the mitochondria, and die
C. The organism would survive, but would be weak
3. Loose heme can have a damaging effect on tissues. The iron ion in the heme is very reactive, and can create free radicals. These ions disrupt systems and stop cellular processes. Which of the following would cause excessive loose heme?
A. Damage to the muscles
B. Mutated enzymes responsible for processing heme
C. Drinking too much coffee
References
- Lodish, H., Berk, A., Kaiser, C. A., Krieger, M., Scott, M. P., Bretscher, A., . . . Matsudaira, P. (2008). Molecular Cell Biology(6th ed.). New York: W.H. Freeman and Company.
- Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company.
- Pough, F. H., Janis, C. M., & Heiser, J. B. (2009). Vertebrate Life. Boston: Pearson Benjamin Cummings.
Heme
No comments:
Post a Comment