Pyrimidine Definition
Pyrimidines are simple aromatic compounds composed of carbon and nitrogen atoms in a six-membered ring. The term pyrimidine is also used to refer to pyrimidine derivatives, most notably the three nitrogenous bases that, along with the two purines, are the building blocks of both deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The pyrimidine nitrogenous bases are derived from the organic compound pyrimidine through the addition of various functional groups. The three pyrimidines are thymine which is only found in DNA, uracil which is only found in RNA, and cytosine which is found in both DNA and RNA.
Pyrimidine Structure
Pyrimidine is a simple aromatic ring composed of two nitrogen atoms and four carbon atoms, with hydrogen atoms attached to each carbon. The carbon and nitrogen atoms are connected via alternating double and single bonds. This bond structure allows for resonance, or aromaticity, causing the ring to be very stable. There are many derivatives of this structure through the addition of one or more functional group. These derivatives all retain the simple six-membered ring, but the modifications can range from addition of a few atoms in nucleic acids to complex structures in drugs and vitamins.
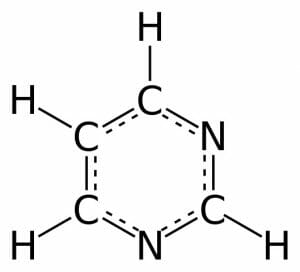
This figure depicts the 2-dimensional structure of a pyrimidine molecule. The atoms can be numbered counter-clockwise from the bottom N.
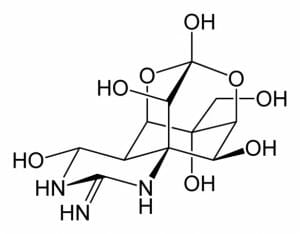
This figure depicts the complex structure of tetrodotoxin, a pyrimidine derivative. The pyrimidine ring is found in the lower left.
Structure of Nitrogenous Bases
The three pyrimidine nitrogenous bases, thymine (T), cytosine (C), and uracil (U), are modified forms of the aromatic compound pyrimidine. They consist of a six-membered ring with two nitrogen atoms and four carbon atoms, but instead of being an aromatic ring with alternating double and single bonds they all have a ketone (carbonyl group) on the 2′ carbon atom (the carbon between the two nitrogen atoms). The addition of this double bond removes a bond from the ring, resulting in two double bonds and four single bonds.
In addition to the carbonyl group, the three nitrogenous bases also have a functional group attached to the 4′ carbon (a ketone for T and U, and an amino group for C), and T has a methyl group attached to the 5′ carbon as well. The addition of another ketone in T and U removes another double bond from the ring, leaving only one double bond in U and T, and two double bonds in C. In all three there are only two bonds to the 1′ nitrogen; this is where the nitrogenous base attaches to the sugar in the nucleic acid to form a nucleoside (or a nucleotide when phosphorus is attached).
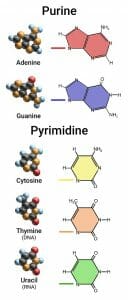
This figure depicts the structure of the five nitrogenous bases separated into purines and pyrimidines. The colored line is where the base attaches to the ribose sugar.
A number of modified pyrimidines can also be found in both DNA and RNA. The nucleotides can be altered through oxidation, methylation, amination, or the addition of other functional groups such as aldehydes, thioketones, and alcohols These modifications often result in deleterious effects such as altering gene expression or disrupting replication. Modifications are more prevalent in RNA than DNA, particularly in small nuclear RNA (snRNA).
Pyrimidine Function
The aromatic compound pyrimidine, and its derivatives, are ubiquitous in nature. They are found in nucleic acids, vitamins, amino acids, antibiotics, alkaloids, and a variety of toxins. These derivatives play a variety of functions, from production of amino acids and proteins, contributing to an organisms’ health, providing vital nutrients, boosting the immune system, or antagonising and destroying cells. For example, the neurotoxin tetrodotoxin is a pyrimidine derivative. It is found in a number of species including the Japanese puffer fish, the blue-ringed octopus, and the orange-bellied newt. Tetrodotoxin prevents the transmission of nerve signals and can result in paralysis and death.
Pyrimidine derivatives also play an important role in drug development, either in concert with other compounds or on their own. They have been used in a wide variety of pharmaceuticals including general anesthetics, anti-epilepsy medication, anti-malaria medication, drugs for treating high blood pressure, and HIV medication.
Function of Nitrogenous Bases
The three pyrimidine nitrogenous bases, along with the two purine bases, act as the genetic material in all living organisms. Their function is two-fold: to pass information from parent to offspring through replication, mitosis, and meiosis, and between different organisms through horizontal gene transfer; and to encode genes and regulatory information.
Before DNA can be passed from parent to offspring, it must first be passed on to daughter cells. The nucleic acids pass on information via semi-conservative replication. This takes advantage of the fact that there are strict rules in the way in which the nitrogenous bases pair with each. In what is known as Chargaff’s rules, the pyrimidines, which are single-ringed molecules, will each bind with a double-ringed purine. This allows any double-stranded DNA to maintain a constant width along the length of the molecule. The pairings are even more specific than a pyrimidine with a purine – cytosine will only bind with guanine, and thymine and uracil will both only bind with adenine. This is because cytosine and guanine both have the ability to form three hydrogen bonds, while the other three bases can only form two hydrogen bonds. These hydrogen bonds are what holds the bases, and thus the strands, together. During DNA replication a parent molecule acts as a template. It is then copied by the formation of an anti-parallel strand that forms according to Chargaff’s rules.
The nitrogenous bases, and the nucleotides which they are a part of, form strands of DNA and RNA which are composed of coding and non-coding regions. The coding regions can be translated into amino acids which form proteins. This is done through transcription, or the formation of an RNA intermediary, followed by translation, the reading of the messenger RNA (mRNA) to form peptide chains. While the non-coding regions are not transcribed, they have a variety of important functions including regulation, and encoding molecules such as ribosomal RNA (rRNA) or transfer RNA (tRNA), both of which are further involved in translation and gene expression.
Quiz
1. How many carbon atoms are in a pyrimidine ring?
A. two
B. three
C. four
D. six
2. Which is not a function of pyrimidine?
A. hereditary material
B. energy source
C. anti-epilepsy drugs
D. vitamin B
3. What pyrimidine is not found in DNA?
A. thymine
B. adenine
C. cytosine
D. uracil
4. Which nitrogenous base does uracil bind to?
A. thymine
B. adenine
C. cytosine
D. guanine
References
- Brown, T. (2012).Introduction to genetics: a molecular approach Ch. 2. New York, NY: Garland Science, Taylor & Francis Group, LLC. ISBN: 978-0-8153-6509-9.
- Lagoja, I. M. (2005). “Pyrimidine as constituent of natural biologically active compounds”. Chem. Biodiversity 2: 1–50.
Pyrimidine
This is a really informative knowledge, Thanks for posting this informative Information. Pyrimidine
ReplyDelete