Antibody Definition
An antibody is a specialized defense protein synthesized by the vertebrate immune system. These small structures are actually made of 4 different protein units. The ends of the molecule are variable, and can be adapted to bind to any molecule. The shape is determined by the antigens in the system which are causing damage. Special immune cells detect these antigens and create a reciprocal antibody. This generalized structure is repeated many times, to flood the system with antibodies. These proteins bind to and surround the antigens, preventing further spread or infection.
It is in this way that an organism can identify “self” from “non-self”. For instance, the surface of bacterial cells has certain proteins and carbohydrates, which can be identified by the immune system. B lymphocytes, a special immune cell, create and release antibodies which attack the invading bacteria. An antibody attached to a bacteria not only prevents it from completing normal processes, but helps direct white blood cells to eat the bacteria. These macrophages, as they are known, identify food based on the tail end of the antibody.
In the blood, antibodies account for around 20% of the total protein. This is a very significant amount. Although a single antibody can be very small, an organisms must have many antibodies to fight the many types of antigens present in the system. Further, many of each type is needed. It often takes many antibody molecules to target and identify a large bacteria. Viruses, while they are smaller, are much more abundant and need equal amounts of antibody to quell.
While other organisms often have immune systems based on similar concepts, the term antibody and the structure described below are unique to mammals. An antibody may also be referred to as immunoglobulin, a term which describes a protein used in an immune function. The most common antibody is Immunoglobulin G (IgG) in mammals. Antibodies, if they exist, are not well understood in invertebrates and plants. While it is known that these organisms also have immune systems, it is not entirely clear how they function.
Antibody Structure
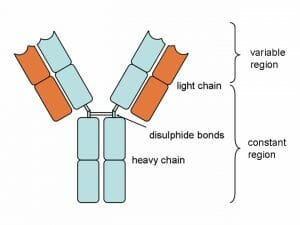
Above is a typical antibody. Notice that the structure is actually made of 4 different protein chains. There are two heavy chains and two light chains. The two heavy chains are connected by a disulfide bond, which exists between two sulfide atoms present in the amino acids of each chain. The light chains attach to the sides of the heavy chain, through a series of non-covalent bonds and weak interactions.
Each chain is broken into two regions, the constant region and the variable region. The constant region is produced directly from the DNA, and is the same on all antibody molecules of the same type. The variable region is the part of the antibody which changes according to the antigen present. The B lymphocytes are in charge of a complex process which matches the variable region to the antigen, and then mass produces the correct antibody.
It is the variable region that has a binding site, capable of binding to the antigen. The binding site is specific in that it is designed to attach only to the intended antigen. It does this by being as compatible to the antigen as possible. If the antigen is hydrophobic, so is the binding site. If the antigen is negatively charged, the binding site will optimally be positively charged to help bind the antigen. Further, the entire shape of the antibody head is specifically formed to the shape of the antigen. This ensures that the antibody will be specific to the antigen. The constant region of the antibody can come in a number of forms, and can be assembled into larger complexes with different shapes.
Antibody Action in Autoimmune Disease
In some cases, the antigen is so close to a molecule produced the organism that the immune system ends up attacking itself. This is known as an autoimmune disease. The immune system, upon being presented with an antigen, forms a defense. In these cases, the antigen is usually a protein. The protein is similar to a protein produced by the organism. While the antibody forming system can be very specific, it cannot accurately identify two molecules which have the same shape. Thus, even if the molecules are actually “self” it can end up attacking them.
Autoimmune diseases can be caused by a number of conditions. Some include viruses, such as HIV, which make the immune system target itself. Still other autoimmune diseases, such as certain forms of diabetes, can be caused by the immune system attacking the pancreas, an insulin-secreting organ. Some research has been done which may link this autoimmune reaction to the proteins found in animal products. While plant proteins have evolved on a completely different trajectory, humans and farm animals share many of the same genes. This means that they produce many of the same proteins. If these proteins leak into the body without being broken down, they could be identified as an antigen.
Upon seeing this antigen, the immune system will create an antibody, to contain it. These antibodies will be mass produced, and sent all over the body to attack any protein of the same shape. This can cause a serious problem for your body. Let’s say you just had a hot dog. All parts of a pig and cow are used to create hot dogs. Needless to say, you will likely get proteins which originated in the animal’s pancreas, cartilage, or other organs. Because their proteins are so similar to yours, your body will begin to have an immune reaction in places these proteins are present. This could be a major cause of diseases like diabetes, arthritis, and possibly even conditions like Multiple Sclerosis.
Antibody Use in Analytical Techniques
An antibody can be a very useful tool in the laboratory, as well. Because an antibody is so specific, and binds tightly in certain conditions, antibodies are used in a number of applications used to filter a solute from a solution. In column chromatography, they are used to bind to solute molecules passing by. As the solution is drained off, the antibody retains the solute. A different solution, with a different pH, can be washed over the antibody media, and the antibody will change shape and release the solute.
Another common use of an antibody in the laboratory is to detect certain substances. An antibody is attached to another protein, used to create a visible molecule. When the antibody is in the presence of the antigen, the antibody changes shape and activates the enzyme. This action creates visible molecules, and can be detected either visually or through a computer. This allows scientist to detect very small samples for a substance, relatively cheaply. This can be used to diagnose disease, test products, and test the safety of consumer products.
Quiz
1. A scientist has an antibody specific to guanine, an amino acid. He puts the antibody in a column, and pours in a mixture of guanine, taurine, and adenine. The solution is drained off into Beaker 1. A new acidic solution is placed in the column. The acid changes the shape of the antibody. The solution is drained off into Beaker 2. Where is the guanine?
A. Beaker 1
B. Beaker 2
C. In the Column
2. In some autoimmune diseases, sometimes a treatment includes destroying parts of the immune system. How can this help?
A. With the cells destroyed, no antibodies can be produced
B. This cannot be helpful
C. With no immune system, the body cannot be overwhelmed by bacteria
3. When scientists first learned about bacteria and germs, sterilization was the resounding call. Why is this method being rethought?
A. The immune system needs practice
B. An antibody cannot form without severe disease
C. Producing less antibodies makes you healthier
References
- Campbell, T. C., & Campbell, T. M. (2006). The China Study. Dallas: Benbella Books.
- Nelson, D. L., & Cox, M. M. (2008). Principles of Biochemistry. New York: W.H. Freeman and Company.
- Pough, F. H., Janis, C. M., & Heiser, J. B. (2009). Vertebrate Life. Boston: Pearson Benjamin Cummings.
- Widmaier, E. P., Raff, H., & Strang, K. T. (2008). Vander’s Human Physiology: The Mechanisms of Body Function (11th ed.). Boston: McGraw-Hill Higher Education.
Antibody