The effects of isotonic, hypotonic, and hypertonic extracellular environments on plant and animal cells is the same. However, due to the cell walls of plants, the visible effects differ. Although some effects can be seen, the rigid cell wall can hide the magnitude of what is going on inside.
Osmosis and Diffusion
Osmosis has different meanings in biology and chemistry. For biologists, it refers to the movement of water across a semipermeable membrane. Chemists use the term to describe the movement of water, other solvents, and gases across a semipermeable membrane. Both biologists and chemists define diffusion as the movement of solute particles (dissolved materials) from an area of higher concentration to lower concentration until equilibrium is reached.
How Osmosis Works
Osmosis is a passive transport system, meaning it requires no energy. It causes water to move in and out of cells depending on the solute concentration of the surrounding environment. This movement is caused by a concentration gradient created when there are different solute concentrations inside and outside the cell. It doesn’t matter what dissolved materials make up the solute, only the overall concentration. It is important to note that cells do not regulate the movement of water molecules in and out of their intracellular fluid. They rely on other systems in the body (such as the kidneys) to provide an isotonic external environment (see below).
Isotonic Solution
A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations inside and outside are the same (iso means equal in Latin). In this state there is no concentration gradient and therefore, no large movement of water in or out. Water molecules do freely move in and out of the cell, however, and the rate of movement is the same in both directions.
Hypotonic Solution
A hypotonic solution has a lower solute concentration than inside the cell (the prefix hypo is Latin for under or below). The difference in concentration between the compartments causes water to enter the cell. Plant cells can tolerate this situation better than animal cells. In plants, the large central vacuole fills with water and water also flows into the intercellular space. The combination of these two effects causes turgor pressure which presses against the cell wall causing it to bulge out. The cell wall helps keep the cell from bursting. However, if left in a highly hypertonic solution, an animal cell will swell until it bursts and dies.
Hypertonic Solution
In Latin, the prefix hyper means over or above. Hypertonic solutions have a higher solute concentration than inside the cell. This causes water to rush out making the cell wrinkle or shrivel. This is clearly seen in red blood cells undergoing a process called crenation. Plant cells in a hypertonic solution can look like a pincushion because of what’s going on inside. The cell membrane pulls away from the cell wall but remains attached at points called plasmodesmata. Plasmodesmata are tiny channels between plant cells that are used for transport and communication. When the inner membrane shrinks, it constricts the plasmodesmata resulting in a condition called plasmolysis.
Comparison Chart
| Isotonic Solution | Hypotonic Solution | Hypertonic Solution | |
|---|---|---|---|
| High level of solutes outside of the cell | No | No | Yes |
| Low level of solutes outside of the cell | No | Yes | No |
| Water movement depends on the type of solute | No | No | No |
| If uncontrolled, may lead to cell death | No | Yes | Yes |
| Can cause the cell to wrinkle/shrivel | No | No | Yes |
| Can cause the cell to swell/burst | No | Yes | No |
| In plants, results in plasmolysis | No | No | Yes |
| In plants, results in turgor pressure inside the cell | No | Yes | No |
| Causes water movement via osmosis | No | Yes | Yes |
| Represents a homeostatic state | Yes | No | No |
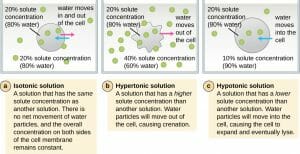
The image above shows what happens to a cell in isotonic, hypertonic, and hypotonic solutions.
References
- OpenStax College. (2018). Anatomy & Physiology. Houston, TX. OpenStax CNX. Retrieved from http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.119
- Tonicity. (n.d.). In Wikipedia. Retrieved April 17, 2018 from https://en.wikipedia.org/wiki/Tonicity
Isotonic vs. Hypotonic vs. Hypertonic Solution
No comments:
Post a Comment