Definition of Exergonic Reaction
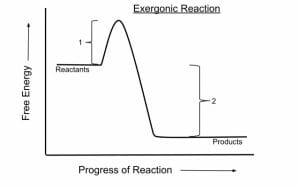
An exergonic reaction is a reaction that releases free energy. Because this type of reaction releases energy rather than consuming it, it can occur spontaneously, without being forced by outside factors.
In chemistry terms, exergonic reactions are reactions where the change in free energy is negative. Free energy measures the total amount of energy available in a system; negative changes mean that energy has been released, while positive changes mean that energy has been stored.
Reactions where chemical bonds are broken, releasing the energy in those bonds, are often exergonic reactions. These reactions where chemicals are broken down are called “catabolism” – the destructive part of metabolism.
By contrast, reactions where chemical bonds are formed are often endergonic. In these constructive reactions where complex molecules are created, the organism uses energy harvested from photosynthesis or cellular respiration and puts that energy into chemical bonds. These creative parts of metabolism are called “anabolism.”
For living things, the chemical bonds in molecules such as sugars, proteins, and fats can be used as energy storage.
This can be seen in metabolism, where sugars, proteins, and fats are created by consuming energy from photosynthesis or cellular respiration. It can be seen again when the organism needs energy and these molecules are broken down.
Fun fact: fat contains more energy than sugar by weight because fat molecules contain more chemical bonds than sugar molecules. The more bonds the molecule contains, the more energy can be released by breaking those bonds!
Although the breakdown of sugars, fats, and other exergonic reactions are spontaneous – living things use enzymes to speed these reactions up tremendously.
The enzymes work by bringing the substrate molecule (such as a fat or sugar to be metabolized) into an ideal arrangement for the reaction to begin. This lowers the activation energy of the exergonic reaction, making it much more likely to occur.
Functions of Exergonic Reactions
Exergonic reactions are used by living things to move energy out of “storage” in one molecule, such as a sugar or fat, and into an active form such as ATP. This is done by breaking the chemical bonds in the sugar or fat, and passing its energy in the form of electrons or another currency to a new molecule.
The highly efficient process of cellular respiration uses electron transport chains and other highly specialized chemical equipment to create a shocking 38 molecules of ATP from a single glucose molecule (although six molecules of ATP are consumed in the process, for a net gain of 32).
Less efficient organisms may only be able to harness enough energy from the breaking of glucose’s bonds to produce a few molecules of ATP – but this is still sufficient to sustain life!
Examples of Exergonic Reactions
Glycolysis
Glycolysis is the first process used by prokaryotes and eukaryotes alike to turn energy stored in sugar into ATP. For eukaryotes, glycolysis is only the first step in a process that leads to cellular respiration; for prokaryotes, glycolysis may be the only means they have of obtaining ATP from glucose.
The term “glycolysis” comes from the roots words “glyco” for sugar and “lysis” for “to split.” It literally means “splitting sugar” – and that is exactly what happens in glycolysis, where a molecule of glucose is split into two molecules of pyruvate.
By breaking the chemical bonds that held the two molecules together as one, the enzymes of glycolysis harvest enough energy to produce two molecules of ATP.
Sugars are a good form of energy storage for cells because they are fairly stable; unlike ATP, they do not spontaneously decay and release their energy every time they run into an enzyme that needs energy. Controlling the rate of glucose to ATP conversion allows the cell to control the rate at which it spends the energy it has stored. This can be a lifesaving adaptation in times when food is scarce.
Cellular Respiration
In eukaryotic cells that practice cellular respiration, the pyruvate molecules left over from glycolysis undergo even more bond-breaking to release even more energy.
The bond-breaking equipment of cellular respiration is so advanced that at the end of this process, all that’s left is carbon dioxide. Glucose has been broken down into single-carbon units!
The energy released by this reaction is harvested to produce a net gain of 30 more molecules of ATP, in addition to the two gained from gycolysis.
Fatty Acid Catabolism
Fatty acid catabolism refers to the breakdown of fatty acids.
For organisms that can afford long-term energy storage, fatty acids are a great way to do it. Fat molecules can contain much more energy than sugar molecules, because they contain many more chemical bonds.
While glucose molecules contain 6 carbon atoms, 6 oxygen atoms, and 12 hydrogen atoms bound together – fatty acids contain anywhere from 2 to 26 carbon molecules, and up to 2 hydrogen atoms per carbon.
In fatty acid catabolism, these long energy-storing chains are broken down into smaller chunks that can be broken down into carbon dioxide, just like with glucose in cellular respiration.
And just like the conversion of glucose to ATP, controlling the rate of fatty acid catabolism allows organisms to control how fast they use stored energy!
Quiz
1. Which of the following is LEAST likely to be an exergonic reaction?
A. The splitting of glucose into pyruvate
B. The splitting of a protein into amino acids
C. The synthesis of a starch from several molecules of sugar
D. The breakdown of a toxin into two non-toxic components
2. Which of the following might stop the production of ATP within a cell?
A. Flooding the cell with ATP, pushing the equilibrium toward the reactant side of the equation.
B. Drastically lowering the temperature so that chemical reactions proceed at a slower rate.
C. Removing the enzymes which lower the activation energy for the reactions that create ATP.
D. All of the above.
3. Which of the following is NOT a reason why animals use fatty acids for energy storage?
A. Fatty acids contain a large number of chemical bonds, allowing them to store more energy than sugar.
B. Control of fatty acid anabolism and catabolism allows the organism to “decide” how quickly to spend its stored fuel.
C. Fatty acids are weigh less than sugars containing the same amount of calories, allowing the organism to carry more stored energy with less effort.
D. All of the above.
References
- Metabolism and energy. (n.d.). Retrieved April 29, 2017, from http://www.rsc.org/Education/Teachers/Resources/cfb/metabolism.htm
- MacNaught, A. D., & Wilkinson, A. (1997). Compendium of chemical terminology: IUPAC ecommendations. Oxford: Blackwell Science.
- Stryer, L., Tymoczko, J. L., & Berg, J. M. (2002). Biochemistry. New York: W.H. Freeman.
- Pawar, P., M., & R. (2013, October 14). Oxidation of Fatty Acids – via Beta-Oxidation | Biochemistry Notes. Retrieved April 29, 2017, from http://pharmaxchange.info/press/2013/10/oxidation-of-fatty-acids/
Exergonic Reaction
No comments:
Post a Comment